Abstract
INTRO: Primary hemophagocytic lymphohistiocytosis (pHLH) comprises a group of severe hyperinflammatory disorders associated with germline mutations affecting PRF1, UNC13D,STX11 and STXBP2 - genes essential for perforin-dependent lymphocyte cytotoxicity. Consequently, humans and mice with pHLH exhibit reduced or absent lymphocyte killing. This killing function is critical for normal immunoregulation, as is evident in perforin-deficient mice, where failure of CD8 T cells to eliminate activated antigen-presenting cells leads to prolonged and excessive T cell activation and pro-inflammatory cytokine production. The immunoproteasome plays essential roles in processing and presentation of antigens and associated immune effector cell functions, especially in the setting of inflammation. We thus hypothesized that immunoproteasome inhibition might lessen inflammation in HLH. Despite current treatments, up to 50% of patients with HLH die due to refractory disease or the complications of its treatment and therefore it is critical to develop safer and more effective therapies.
METHODS: To test this hypothesis, we first developed an in vitro model of HLH in which splenocytes from P14 mice (which carry a T cell receptor specific for the Lymphocytic Choriomeningitis virus (LCMV) gp33 peptide) were labelled with carboxyfluorescein succinimidyl ester (CFSE), treated or not with the immunoproteasome inhibitor ONX-914, and infected with LCMV. Three days later, cells were pulsed for 4 hours with gp33 peptide, harvested, and evaluated for T cell proliferation and T cell intracellular interferon-gamma (IFNg) and tumor necrosis factor alpha (TNF) production. To examine the effects of immunoproteasome inhibition in vivo, LCMV-infected perforin-deficient (Prf1-/-) mice were treated with intravenous KZR-616 (zetomipzomib; an immunoproteasome inhibitor equivalent to ONX-914 but optimized for in vivo use) on varying days after LCMV infection. On days 8-9 post infection, organ size, immune cell subsets, serum cytokine levels, and lymphocytic tissue infiltration were assessed. Immunoproteasome subunit inhibition was measured via Proteasome Constitutive-Immuno Subunit ELISA (ProCISE) in the kidney, liver, and spleen at various time points after drug administration in LCMV-infected Prf1-/- mice compared to C57BL/6 wildtype controls.
RESULTS: Immunoproteasome inhibition significantly reduced in vitro LCMV-induced P14 CD8 T cell proliferation and IFNg production and it did so in a dose dependent manner. In contrast, there was no reduction in P14 CD8 T cell production of TNF. Untreated LCMV-infected animals developed organomegaly with increased frequencies and absolute numbers of intrasplenic and intrahepatic T cells and neutrophils. Further, they exhibited significantly elevated serum IFNg and TNF levels. Following treatment with KZR-616, LCMV-infected mice exhibited significantly reduced organomegaly, fewer tissue infiltrating T cells and neutrophils, lower serum IFNg levels and less IFNg production following ex vivo gp33 re-stimulation. Consistent with findings from our in vitro LCMV infection model, there was no reduction in serum TNF levels or in the frequency or number of CD8 T cells producing TNF upon gp33 restimulation. Successful inhibition of immunoproteasome subunit activity was achieved in all tested organs within 4 hours of KZR-616 administration in both LCMV-infected Prf1-/- and C57BL/6 mice, without significant differences. Immunoproteasome subunit activity gradually recovered to levels of LCMV-infected vehicle-treated controls by 5 days after a single KZR-616 dose.
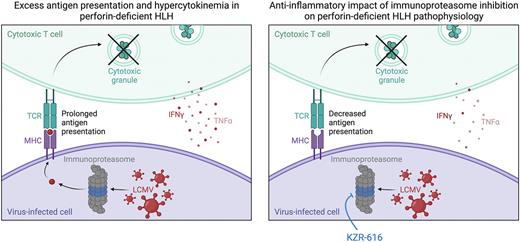
CONCLUSION: Immunoproteasome inhibition represents a rational and potentially beneficial therapeutic strategy for pHLH with in vitro and in vivo exposure to the selective immunoproteasome inhibitors ONX-914 and KZR-616 lessening many disease manifestations. The anti-inflammatory effect of immunoproteasome inhibition in our model of pHLH is summarized in the Figure, whereby KZR-616 is proposed to decrease presentation of LCMV antigens leading to less T cell activation, proliferation and IFNg production. The lack of impact on TNF production might explain why some disease manifestations are not improved in these studies. Hence, experiments are ongoing to optimize the anti-inflammatory effects of immunoproteasome inhibition by combining KZR-616 with agents that block the effects of TNF.
Disclosures
Anderl:Kezar Life Sciences: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Kirk:Kezar Life Sciences: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Nichols:Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal